News
NAFDAC To Nigerians: Why You Should Avoid These Two ‘ Contaminated’ Cough Syrups
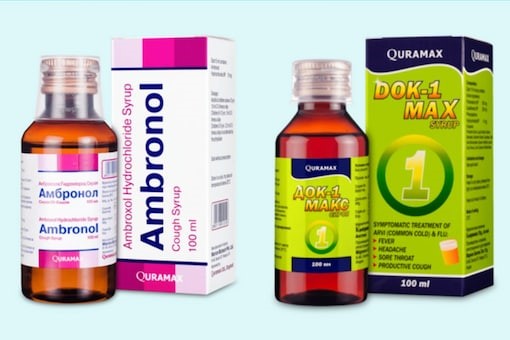
-
NAFDAC said the two cough syrups were identified in Uzbekistan.
-
The agency identified the products as Ambronol syrup and DOK-1 Max syrup.
-
It said laboratory analysis of samples of both products found the syrups contained unacceptable amounts of certain substances
Eko Hot Blog reports that the National Agency for Food and Drug Administration and Control (NAFDAC) has warned Nigerians against two “contaminated” cough syrups identified in Uzbekistan.
The agency made the warning in a statement issued on Monday.
EDITOR’S PICKS
-
“Pursue Your Passion Without Hesitation” – Eko Hot Blog CEO Makes Passionate Appeal At Staff Retreat
According to NAFDAC, the products are Ambronol syrup and DOK-1 Max syrup.
It noted that the syrups were manufactured by Marion Biotech, India, adding that the manufacturer has not provided guarantees to the World Health Organisation (WHO) on the safety and quality of the products.
The agency also noted that Uzbekistan’s ministry of health carried out laboratory analysis of samples of both products and found that the syrups contained unacceptable amounts of diethylene glycol and ethylene glycol.
NAFDAC warned that diethylene glycol and ethylene glycol can trigger toxic effects in humans.
“Diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal. Toxic effects can include abdominal pain, vomiting, diarrhea, inability to pass urine, headache, altered mental state, and acute kidney injury which may lead to death,” the agency said.
“These substandard products are therefore unsafe and their use, especially in children, may result in serious injury or death.
“NAFDAC implores manufacturers of liquid dosage forms, especially syrups that contain excipients including propylene glycol, polyethylene glycol, sorbitol, and/or glycerin /glycerol, to test for the presence of contaminants such as ethylene glycol and diethylene glycol before use in medicines.
“Although the products are not in the NAFDAC database, importers, distributors, retailers, and consumers are advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the substandard (contaminated) syrups. All medical products must be obtained from authorised/licensed suppliers. The products’ authenticity and physical condition should be carefully checked.”
FURTHER READING
NAFDAC, therefore, advised members of the public in possession of the above-listed products to discontinue sale or use and submit stock to the nearest office of the agency.
Click here to watch our video of the week:
Advertise or Publish a Story on EkoHot Blog:
Kindly contact us at [email protected]. Breaking stories should be sent to the above email and substantiated with pictorial evidence.
Citizen journalists will receive a token as data incentive.
Call or Whatsapp: 0803 561 7233, 0703 414 5611

















