Health
NAFDAC Alerts Nigerians on Counterfeit Cancer Treatment Drug
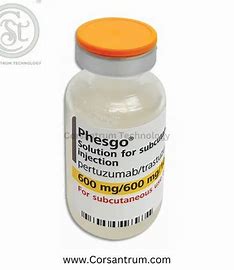
- NAFDAC has warned Nigerians about a fake breast cancer drug, Phesgo 600mg/600mg,
- The counterfeit drug poses serious health risks
- NAFDAC urges the public and healthcare providers to report any suspected counterfeit medicines
The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public alert about a counterfeit cancer treatment drug, Phesgo 600mg/600mg/10ml, associated with batch number C5290S20.
In a statement (No. 051/2024) published on its website, NAFDAC revealed that the alert followed a report from a Lagos University Teaching Hospital (LUTH-NSIA) doctor.
Editor’s Pick
- Marketers Reveal When Petrol Price Will Crash to N500/Per Litre
- Ondo State SSG, Oluwatuyi Passes Away Following Accident
- CHAN: Ogunmodede Calls 26 Players to Eagles Camp in Ikenne
The suspected counterfeit product was brought in by a patient for administration but was fortunately not used.
Upon investigation, Roche, the Marketing Authorisation Holder (MAH), compared the images of the suspected drug to genuine samples. Several discrepancies confirmed the product’s counterfeit status, including mismatched language, missing authentication features, incorrect tamper-evident labels, and a batch number absent from Roche’s database.

Phesgo 600mg/600mg Solution for Injection is a breast cancer treatment drug that kills cancer cells and prevents further growth.

NAFDAC emphasized that counterfeit medicines pose severe health risks as they fail to meet regulatory standards for safety, quality, and effectiveness.
To mitigate risks, NAFDAC has directed its zonal directors and state coordinators to enhance surveillance and remove counterfeit products from circulation.
The agency also urged healthcare providers, distributors, and the public to ensure medical products are obtained only from authorized suppliers and to verify their authenticity.
NAFDAC encouraged the public to report suspected counterfeit drugs through its contact number (0800-162-3322), email ([email protected]), or via its e-reporting platforms, including the Med-Safety app available on Android and iOS devices. Reports of adverse drug reactions can also be sent to [email protected].
Further Reading
- A Rare Medical Phenomenon: Surgeon Contracts Cancer from Patient
- Immigration Made Easy: Germany’s New Visa Portal Goes Live
- Ambode Honored with Community Leadership and Impact Award by CN
This alert has been shared with the World Health Organisation’s Global Surveillance and Monitoring System (GSMS) to enhance international vigilance against counterfeit medicines.
Click HERE For Our Video of The Week
Advertise or Publish a Story on EkoHot Blog:
Kindly contact us at [email protected]. Breaking stories should be sent to the above email and substantiated with pictorial evidence.
Citizen journalists will receive a token as data incentive.
Call or Whatsapp: 0803 561 7233, 0703 414 5611

















