News
COVID-19: Indonesia Launches Human Trials Vaccine With 1,600 Volunteers
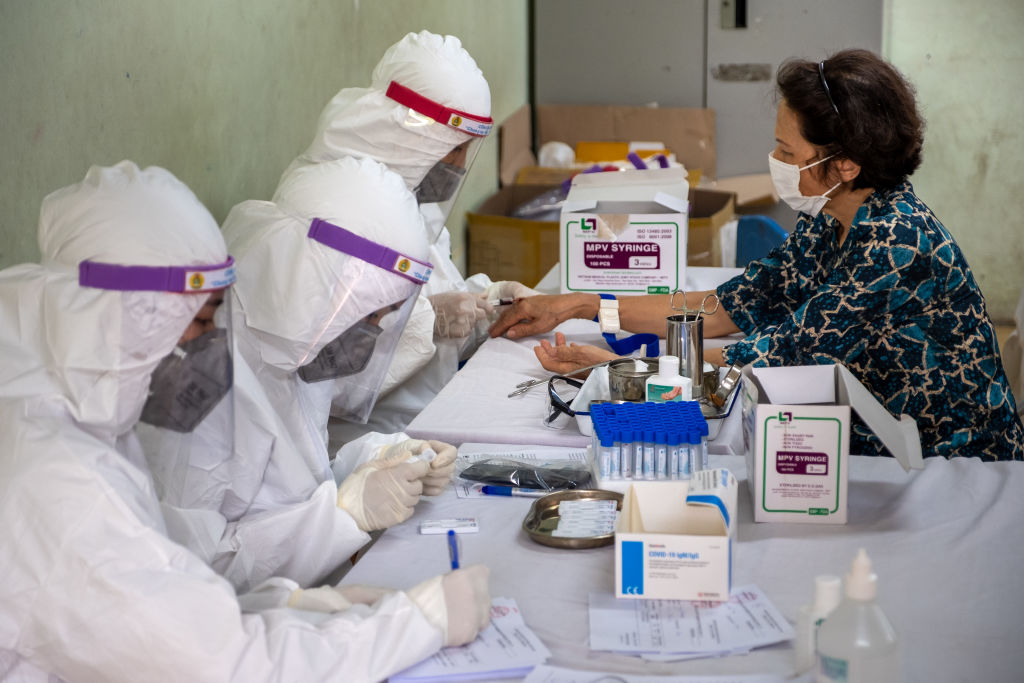
Chinese-made coronavirus vaccine has been launched by Indonesia on Tuesday and it has been announced that 1,600 volunteers will take part in the six-month study.
The vaccine, made by Sinovac Biotech, is among just a few in the world to enter Phase 3 clinical trials, the last step before regulatory approval. It was highlighted that the vaccination will never stop until all citizens of Indonesia takes part.
The treatment, known as CoronaVac, is already being tested on 9,000 health workers in Brazil, the second-hardest-hit country in the coronavirus pandemic after the United States.
Read Also: Lagos Council Bills Churches, Demands Fumigation And Certificate Fees
Indonesia, the world’s fourth most populous country, has been struggling to contain its mounting virus cases, with more than 127,000 confirmed infections and over 5,700 deaths.
The governor of Indonesia’s most populous province, West Java, was among 1,620 volunteers slated to take part in clinical testing, which was set to wrap up in February.
If the vaccine proves safe and effective, Indonesian officials said, there were plans to produce up to 250 million doses for the sprawling archipelago of nearly 270 million, although they gave few details of the tentative roll-out.
On Tuesday, Indonesia’s President Joko Widodo toured a factory in Bandung city, operated by state-owned pharmaceutical firm Bio Farma, where production would begin.
Advertise or Publish a Story on EkoHot Blog:
Kindly contact us at ekohotblog@gmail.com. Breaking stories should be sent to the above email and substantiated with pictorial evidence.
Citizen journalists will receive a token as data incentive.
Call or Whatsapp: 0803 561 7233, 0703 414 5611














